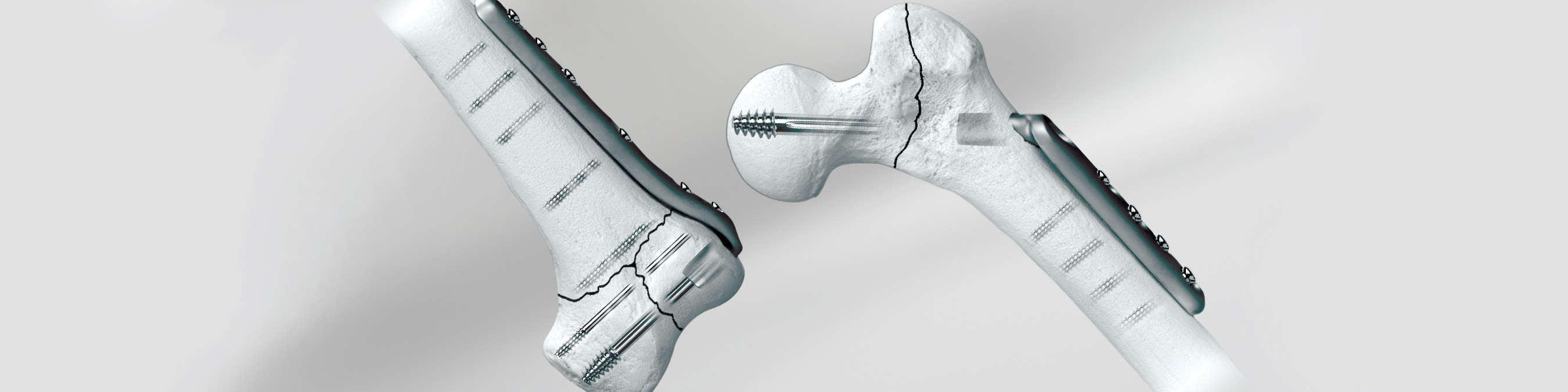
The Versa-Fx® II Femoral Fixation System combines the strength of 22-13-5 stainless steel with a tube/plate junction. Anatomically contoured, the plate is designed provide optimum flexibility in angle fixiation.
A wide selection of sizes, tube/plate angles, and screw hole configurations provide the versatility to use the Versa-Fx II System in a variety of indications, including intracapsular, intertrochanteric, and subtrochanteric fractures (Winquist Type Ill comminuted fractures). The tube plate is available in both keyed and keyless options, with a single set of instruments used for both.
Product Features:
One set of surgically sequenced instruments are available for both keyed and keyless options. System and product features include:
- System offers a variety of tube angles that range between 130°-150° Compression Tube Plate and 95° or 90° Supracondylar Tube Plates
- A choice of three thread options combined with a tapered core helps maximize the bone implant interface
- Screws can be angled up to 30 degrees proximally
- Anatomically contoured side plate provides for flexibility in angle fixation
- Elongated proximal slot for 6.5mm cancellous, 4.5mm cortical, or 7.0mm cannulated screw
- Linked lag screws have sharp leading edge threads for easy insertion, blunt center threads to accommodate a compression screw
- Keyed and keyless tube plate designs
- Pin Relocator provides for accurate reinsertion of Guide Pin