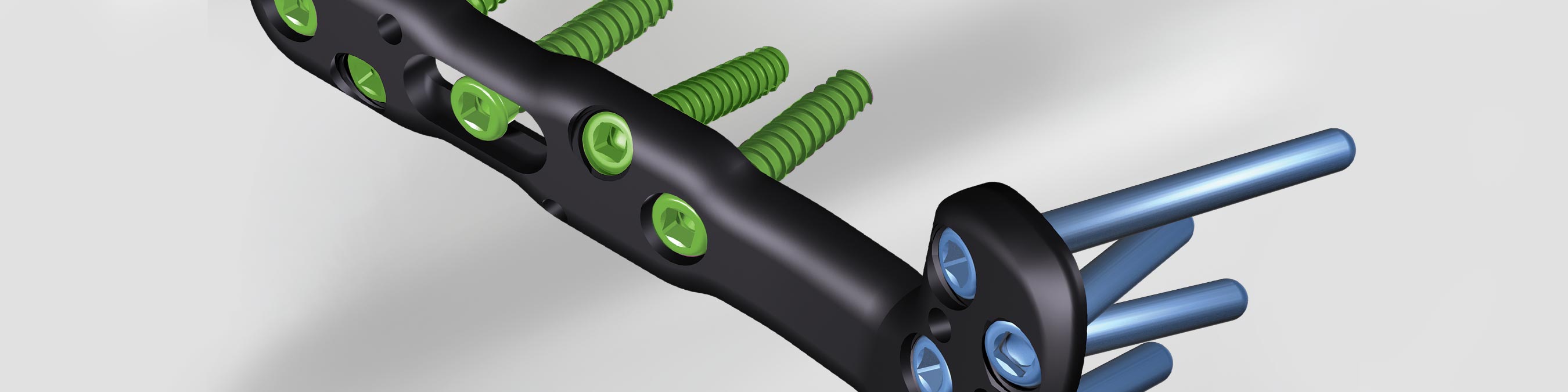
The DVR ® Crosslock Distal Radius Plating System eases the challenge of treating distal radius fractures by incorporating a low-profile, anatomic design with advanced fixation options and streamlined instrumentation. The cross-locking oblique screw options are designed to provide three-dimensional fixation in comminuted fractures and osteoporotic bone.
- Plate is contoured distally to match the watershed line
- Anatomic design of the plate aids in restoring volar tilt
- Narrower shaft makes it easy to fit the plate to the bone without compromising fixation options
- Pegs and locking screws have tapered heads and triple-lead threads to create a stiff construct and to are designed to facilitate insertion and removal
- All locking, non-locking, and oblong holes accept the 2.7mm screws, requiring a single drill bit and driver for efficiency
- Fixed-angle K-wire holes reference screw trajectory and aid the surgeon in optimized plate positioning
- Intersecting proximal and distal pegs in the head create a three-dimensional scaffold to support the articulating surface
- F.A.S.T. Guide ® technology simplifies drilling of fixed angle locking screws