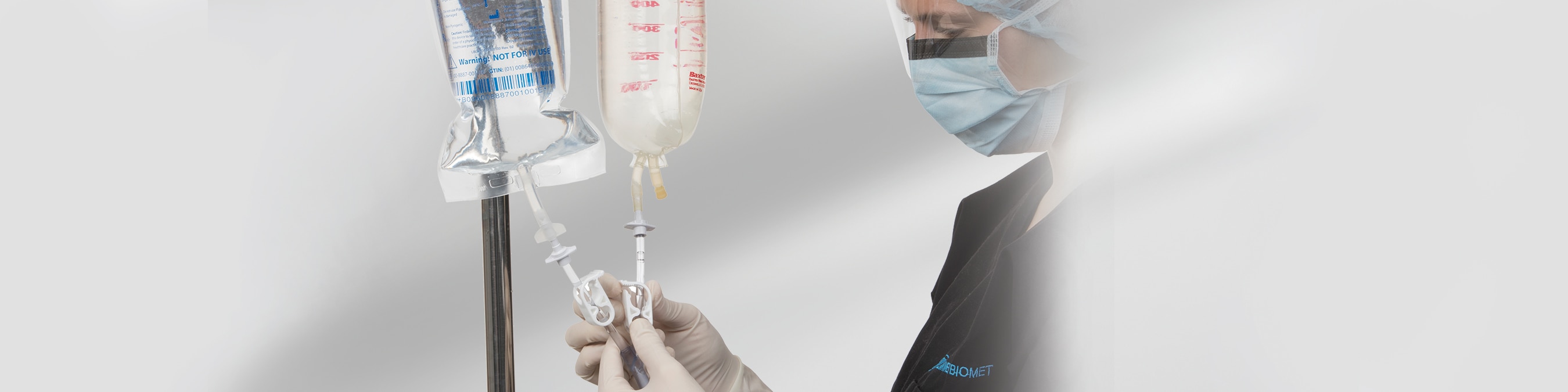
Bactisure Wound Lavage is an aqueous solution used to remove debris, including microorganisms, from wounds using pulsed (jet) lavage. The solution is successful at removing structurally resistant forms of bacteria, including biofilms, on all wound types.
Bactisure™ Wound Lavage
Advancing Biofilm Removal


Active ingredients include ethanol (solvent), acetic acid (pH modifier), sodium acetate (buffer), benzalkonium chloride (surfactant), and water. Product is available in sterile 1000 mL polypropylene plastic bags with an integrated single spike port.

A New Approach to Biofilm Removal
Bacteria can produce Extracelluar Polymeric Substance (EPS) to shield themselves from both mechanical and chemical attack. Bactisure Wound Lavage was specifically designed to remove these structurally resistant forms of bacteria. Our solution physically deconstructs the protective EPS matrix, making bacteria more susceptible to traditional antibiotics and the body’s normal defense mechanisms.
Deconstructs
Bactisure Wound Lavage breaks up crosslinks within biofilm EPS.
Removes
With EPS crosslinks removed, Bactisure Wound Lavage solubilizes biofilms for easy removal via pulsed lavage. Exposed bacteria are subject to lavage removal or inactivation via traditional antibiotics and the body’s normal defense mechanisms.

Indicated for Use on All Wound Types
- Apply just prior to wound closure using Zimmer Biomet Pulsavac® Plus or similar pulsed lavage system.
- Immediately rinse with an equal amount of normal saline using pulsed lavage.
- Not intended for repeated use.
- Not indicated for use during dressing changes or for use by soaking the product into dressings.
- Do not use if there is a history of
allergy to any of the ingredients.

Legal Manufacturer:
Next Science™
8130 Baymeadows Way West Suite 200
Jacksonville, FL 32256
1-855-348-6437