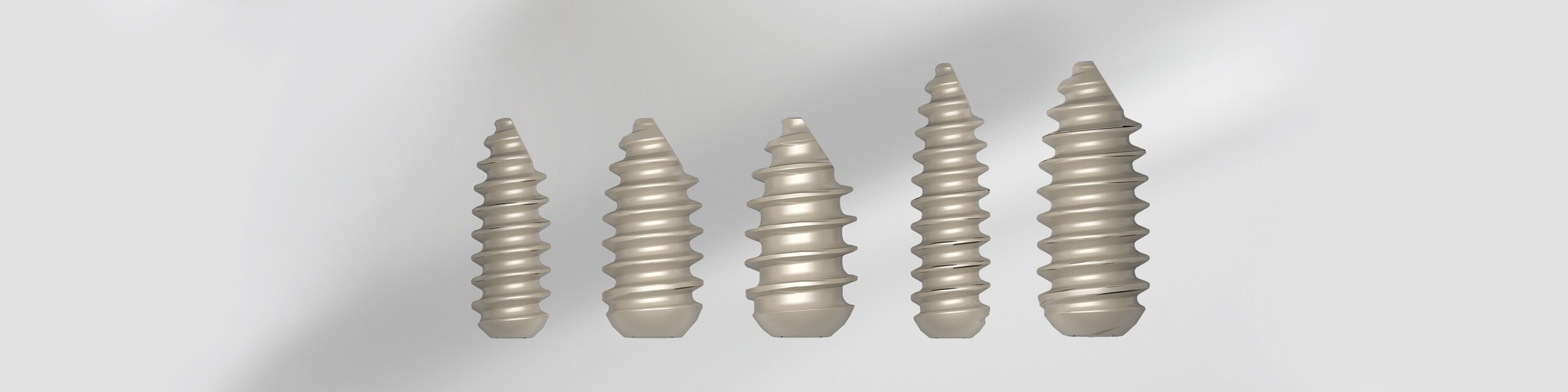
The iFix Interference Screw System was the first self-tapping polyetheretherketone (PEEK) interference screw developed for bone-patellar tendon-bone ACL reconstruction procedures. The interference screws are manufactured using PEEK-OPTIMA®, a non-absorbable polymer that is highly biocompatible, biomechanically strong and radiolucent with decades of proven orthopedic success. With this material and key design features for easy implementation, this technology provides a solution to surgeons and accommodates all types of pathologies and patient needs with multiple sizes.
iFix® Interference Screw

iFix 7-8mm Notching Screw Driver

iFix 9-12mm Notching Screw Driver
- Strong Fixation- Provides high strength for graft fixation
- Self-Tapping- Advanced design eliminates the need for tapping before insertion, which saves surgeons a step in the procedure
- Cannulated Driver- Advanced instrumentation is able to lock and slide on guide wire for easy, aligned insertion of screw
- Radiolucent- Clear post-operative imaging and exceptional image clarity
- Bio-inert- Ideal modulus that is a close match to bone modulus to minimize stress-shielding and provide for a healing environment

PEEK-OPTIMA® is a registered trademark of Invibio Limited.

Legal Manufacturer:
Cayenne Medical, a Zimmer Biomet Company
16597 North 92nd
Street Suite 101
Scottsdale, AZ 85260 USA