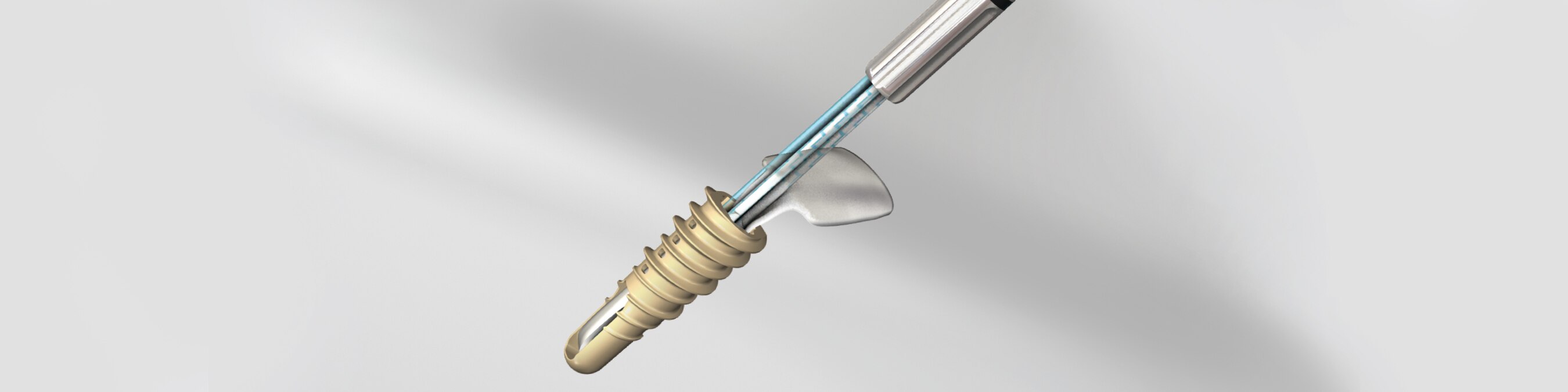
BioWick is the first all-in-one, interpositional scaffold-anchor implant designed to address both the biological and mechanical issues of rotator cuff repair. By combining an interpositional scaffold wick & traditional suture anchor into one implant, BioWick offers a comprehensive approach to repair.
Unique “Interpositional” Scaffold Wick
Arthroscopically deployed at the tendon-bone interface where tendon-bone integration needs to occur.
- Aligned PLGA (85/15 L-lactide/glycolide copolymer) fibers mimic the orientation of collagen fibers in the native tendon1
- 80% porous architecture allows for high permeability
- Bioresorbable material is designed to be replaced by tissue over time