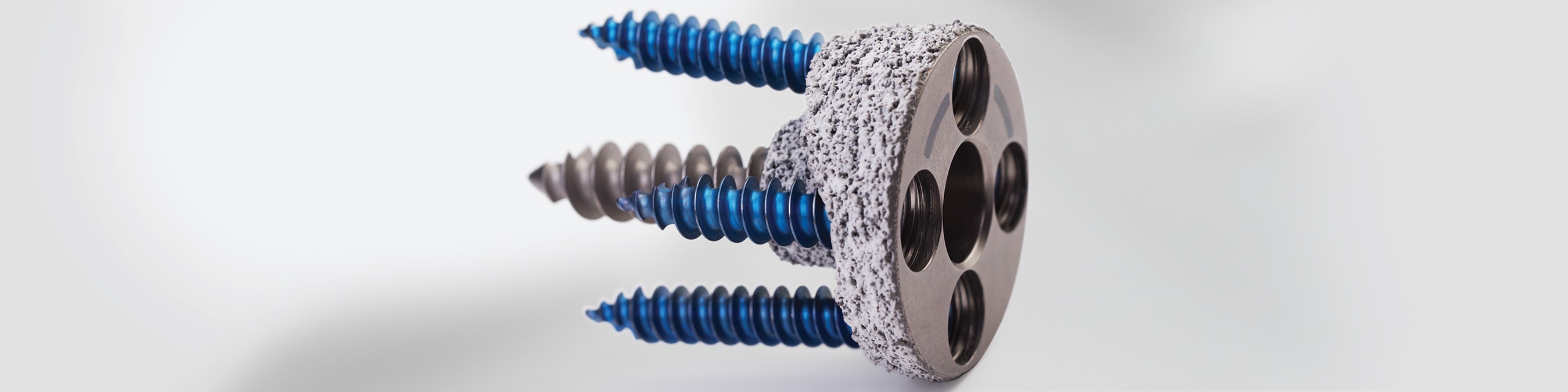
Up to 50% of patients presenting with osteoarthritis and associated rotator cuff arthroplasty have bone erosion that must undergo reconstruction in order to achieve successful shoulder arthroplasty.1 The use of an augmented baseplate provides a simple, reproducible method to restore the glenoid for a stable and successful reconstruction in Reverse Shoulder Arthroplasty. For patients with a lesser degree of bone loss or correction, an augmented baseplate can add benefits of less bone removal, preservation of cortical bone, and lateralization.
Comprehensive® Reverse Shoulder System
Augmented Baseplate


Circular Baseplate allowing the placement of the augment at any location and flexibility to address the full spectrum of glenoid bone wear.
Bone Preservation in cases without glenoid bone and superior augment placement, removes less bone* and lateralizes the glenoid face.
Simplified Surgical Technique adds only three simple steps* to the glenoid bone preparation.
Precise Instrumentation offers reproducible results for optimal augment placement and full bone support.
Alternative to bone grafting and eccentric reaming
*Compared to Comprehensive Mini Baseplate

- Modular 6.5 mm central compression screw for enhanced stability
- Central boss for sharing the sheer load being transferred to the glenoid
- Specialized etch on baseplate face to facilitate accurate augment placement
- Circular Baseplate allows augment to be positioned to any orientation around glenoid face
- HA/TCP over PPS® coating for fixation.

- Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid Morphology in Reverse Shoulder Arthroplasty: Classification and Surgical Implications, Journal of Shoulder and Elbow Surgery 2009 Nov-Dec, 18(6):874-85

Legal Manufacturer:
Biomet Orthopedics
56 East Bell Drive
P.O. Box 587
Warsaw, Indiana 46581 USA