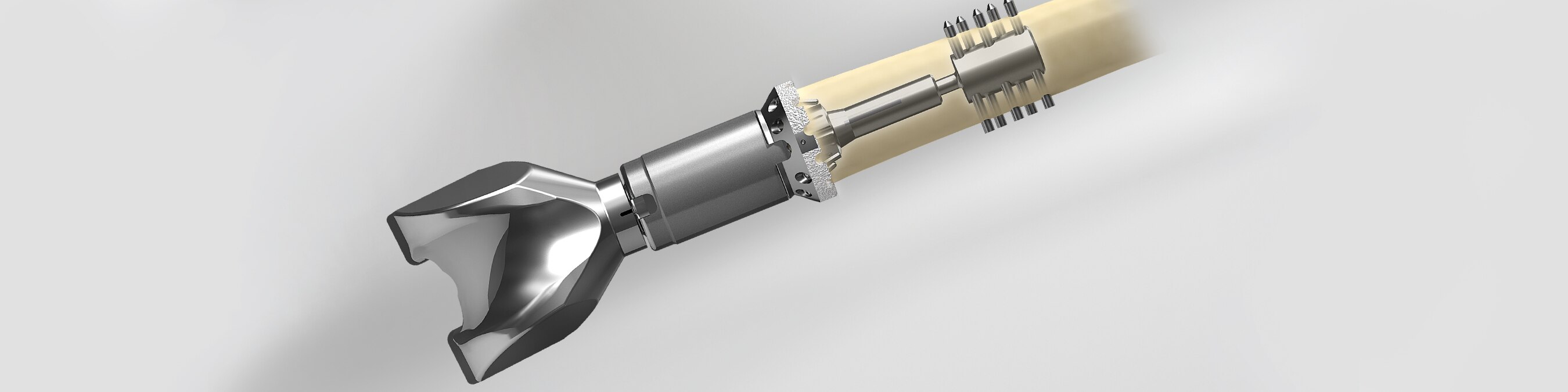
When used in conjunction with the Orthopedic Salvage System or Comprehensive® Segmental Revision System, the Compress Device is designed to replace the distal and/or proximal femur and/or humerus in cases of severe bone loss. The Compress Device exemplifies Wolff’s Law – the principle that bone, when stressed, remodels to become stronger through dynamic bone compression. This creates a stable, bone-implant interface for biologic fixation1-3 and helps to prevent stress shielding.
Compress® Device


- Enhances osseointegration at the bone-implant interface1-3
- Short Compress device offering requires only 65 mm of canal for placement*
- Standard Compress Device offering requires only 100 mm of canal for placement*
- Distal and proximal Reduced Resection Compress bodies offer replacement constructs of 8, 9.5 and 11 cm designed to help retain as much bone as possible
- Standard Resection Compress Device offers a minimal replacement construct of 13 cm when used with the Orthopedic Salvage System
- Compatible with OSS Orthopedic Salvage System components
*Particularly advantageous in massive bone loss cases

- Healey, J. et al. Compress Knee Arthroplasty Has 80% 10-year Survivorship and Novel Forms of Bone Failure. Clinical Orthopaedics and Related Research. 2013 Mar; 471(3): 774-783.
- Zimel, M et al. Revision Distal Femoral Arthroplasty with the Compress Prosthesis Has a Low Rate of Mechanical Failure at 10 Years. Clinical Orthopaedics and Related Research. 2016 Feb; 474(2): 528-536.
- Farfalli, GL et al. Early equivalence of uncemented press fit and Compress femoral fixation. Clinical Orthopaedics and Related Research 2009; 467: 2792-9.

Legal Manufacturer:
Biomet Orthopedics
56 East Bell Drive
P.O. Box 587
Warsaw, Indiana 46581 USA
Authorized Representative:
Biomet UK Ltd.
Waterton Industrial Estate
Bridgend,
South Wales
CF31 3XA
UK