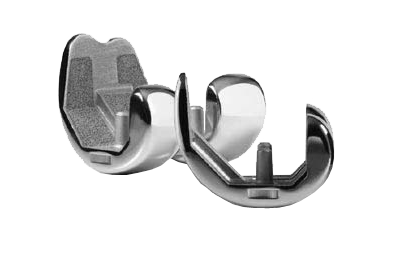
- Zimmer was first to market the innovative asymmetric baseplate design
- Matching the asymmetric tibial shape provides cortical coverage and helps avoid overhang and soft tissue impingement
- Deep, beveled posterior notch helps to prevent impingement of the PCL
- Spiked keel design provides bone-sparing fixation; smooth pegs offer rotational stability
Gender Solutions Natural-Knee Flex
Asymmetric Tibial Trays
Cancellous-Structured Titanium (CSTi™) Porous Coating
- CSTi porous coating option for stable fixation in active patients
- Combines the excellent biocompatibility of titanium with an optimal structure for biological fixation
- Interconnected pores1 resemble human cancellous bone and fine micro-roughness provides enhanced fixation
Ultracongruent Tibial Articular Surface
- Gender Solutions Natural-Knee Flex System includes an ultracongruent tibial articular surface
- This ultracongruent tibial articular surface has published long-term clinical results2 that demonstrate its viability as an alternative to traditional posterior stabilizing designs
- Allows for easy intraoperative conversion from a PCL-retaining to a PCL-sacrificing solution
- Maximum intraoperative flexibility with minimized bone loss
Two Distinct Shapes for Men and Women
- Industry-leading Gender Solutions technology
- Proven success of the Natural-Knee System
- Innovative high-flex designs
- Zimmer was the first to recognize that when it comes to knees, men and women are different. Our groundbreaking research demonstrated that the differences are less about size—and more about shape. Now, Zimmer is applying industry-leading Gender Solutions technology to the clinically successful Natural-Knee System3. The future of total knee arthroplasty is here: the all-new Zimmer Gender Solutions Natural-Knee Flex System.
References
- Bobyn JD, Pilliar RM, Cameron HU, Weatherby GC. The Optimum Pore Size for the Fixation ofPorous-Surfaced Metal Implants by the Ingrowth of Bone. Clin Orthop. 1980, 150:263-70.
- Hofmann A, Tkach T, Evanich C, Camargo P. Posterior Stabilization in Total Knee Arthroplasty With Use of an Ultracongruent Polyethylene Insert. Journal of Arthroplasty. 2000; 15 (5), 576-583.
- Hofmann AA, Evanich, JD, Ferguson, RP, Camargo, MP. Ten- to 14-year clinical follow-up of thecementless Natural-Knee system. Clin Orthop. July, 2001; (388): 85-94.
Technology
Gender Solutions Technology
High Flex Technologies
MIS Instruments
Highly Crosslinked Polyethylene
Congruent and ultracongruent articular surfaces
PSI