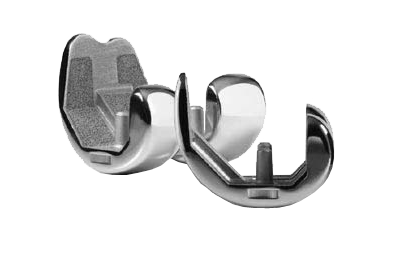
Since 1985, the Natural-Knee System has been used successfully to treat over 500,000 patients.
The Natural-Knee System philosophy is based on Cruciate Retaining (CR) and Cruciate Sacrificing (CS).
The Gender Solutions Natural Knee Flex system is the third generation of evolution for the system and builds on the long clinical lineage the Natural-Knee system while incorporating the Zimmer Gender Solutions and High Flex Technologies.
The Gender Solutions Natural Knee Flex System is designed for use with Natural Knee II Tibia Baseplates. These asymmetrical tibial baseplates provide optimal coverage of the tibial plateau, creating stability without impingement due to overhang, and ultimately replicates natural anatomy.
The Ultracongruent articular surface provides posterior stabilization without bone sacrifice. A deepener trochlear groove prevents excessive load on the patellar component while allowing natural range of motion (ROM).
The asymmetric condyles of the femurs facilitates natural posterior femoral rollback. The lateral condyle moves significantly more posterior than the medial condyle with increasing knee flexion.
The surgery using the Gender Solutions Natural-Knee Flex System creates a true resurfacing by referencing the least involved portion of the femoral condyle, the least- involved portion of the tibial plateau, and the thickest portion of the medial facet of the patella, restoring kinematics and the anatomic joint line.
The Csti™ (Cancellous-Structured Titanium) Porous Coated Implant Interface provides bone in growth.
Posterior Referencing femoral resection results in matched flexion and extension gaps, which help with natural roll back, natural ROM and a potential for reduced polyethylene wear.
The Gender Solutions Natural-Knee Flex system is available with fixed and also rotating platform.
Long-term clinical results confirm that the Natural-Knee System’s design considerations result in improved motion and stability, and promote normal alignment and stable fixation of the implant.
The mean Modified Hospital for Special Surgery knee score improved from 59.0 +/- 13.2 preoperatively to 97.8 +/- 4.7 postoperatively at 10-to-14 years follow-up[5]