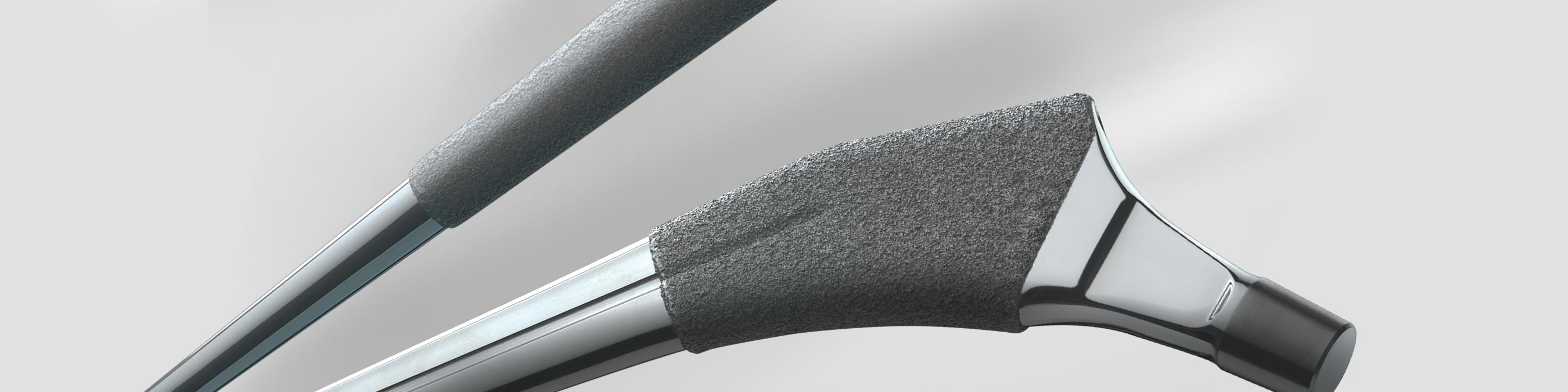
The Zimmer Biomet M/L Taper Hip Prosthesis offers a slim anterior / posterior dimension and bone-conserving alternative for patients, while the collarless prostheiss optimizes the clinically proven tapered wedge fixation philosophy1-3 while providing secure mediolateral stability.
As a combined system, the M/L Taper with Kinectiv® Technology introduces a system of modular stem and neck components designed to help the surgeon optimally restore the hip joint center intraoperatively by addressing leg length, offset, and version independently, while the broad array of neck options efficiently targets a wide range of male and female anatomies.