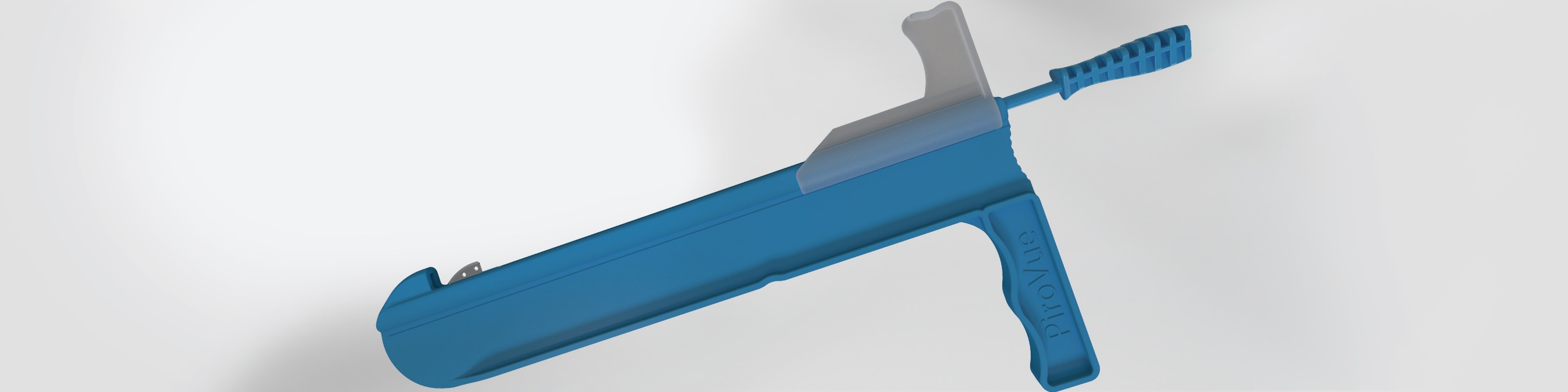
Equinus deformity has been associated with over 96% of biomechanically related foot and ankle pathologies.1
- Gastrocnemius Aponeurosis Intra-Muscular Recession
- Medial mini-open incision (2.5cm-3cm)
- Gastroc intra-muscular recession cadaveric study (using alternative recession instrumentation) showed dorsiflexion improvment.2
- Proximal intramuscular approach avoids the sural nerve3
- Control & guided release
- Versatile
- Recession can be done on both gastrocnemius and soleus muscles as identified by Silfverskiold test2
- Visualization of open procedure
- Convenience of Sterile
Pack Kit
- No endoscopic equipment required
- No ancillary draping time or associated costs