Zimmer Biomet’s PerFuse Percutaneous Decompression Instrument is designed to access the femoral head or humeral head for core decompression. It is intended to be used for the delivery of bone graft material to an orthopaedic site. In addition, PerFuse System facilitates mixing and pre-mixing of bone graft material with I.V. fluids blood, plasma, bone marrow, or other specified blood components deemed necessary by the clinical use requirements.
PerFuse™ Percutaneous Decompression System


Product Features
- PerFuse Cannula creates 6mm hole, which offers post-op flexibility for physician
Simple ease of use:
- Disposable components are always sharp
- 6mm in diameter with two options in length: Large (295mm) and Small (161mm)
- Loaner program lowers facility initial investment
- Disposable tamp allows for multiple intra-operative bone graft options
- Surgical technique eliminates heat generated by power equipment
Instrument Assembly

Reusable Instruments
- PerFuse Handle
- PerFuse Strike Cap
- PerFuse Slide Hammer
- PerFuse Slide Hammer Adapter
- Instrument Case
Disposable Instruments
- PerFuse Cannula
- PerFuse Trocar
- PerFuse Graft Tamp Rod
The full assembly technique and ordering information are available in the PerFuse Instrument Flyer.

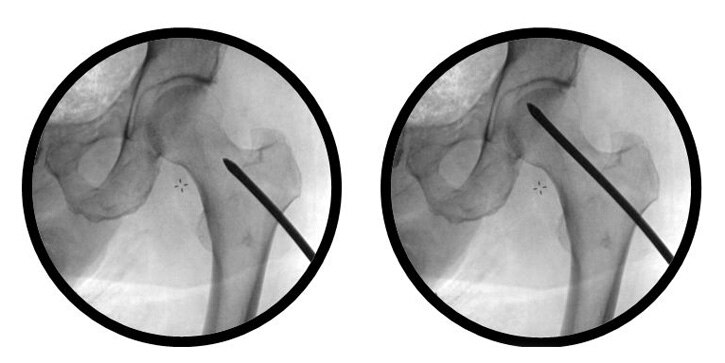
Avascular necrosis (AVN) of the femoral head is a disease commonly associated with corticosteroid use, alcohol abuse, trauma, and sickle cell disease.1 In the United States, between 10,000 and 20,000 people are affected by this pathology annually, aged mostly in their late 30s to early 40s.2
Core Decompression is a surgical technique to treat Avascular Necrosis involving drilling one or more channels into the dead bone (necrotic lesion). Creating a channel into the necrotic lesion is intended to relieve intraosseous pressure within the bone and provide a channel to restore blood flow to the diseased bone.3,4

- Miller, MD, Fischer SJ, Foran JRH. Osteonecrosis of the Hip-OrthoInfo-AAOS. AAOS Sept. 2011. Web. 15 Feb. 2016.
- Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. Journal of the American Academy of Orthopaedic Surgeons 1999; 7(4):250-261.
- Mont MA and Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 1995; 77(3):459-474.
- Van Laere C, Mulier M, Simon JP, Stuyck J, Fabry G. Core decompression for avascular necrosis of the femoral head. Acta Orthop Belg 1998; 64(3): 269-272.

Legal Manufacturer:
Biomet Orthopedics
56 East Bell Drive
P.O. Box 587
Warsaw, Indiana 46581 USA



