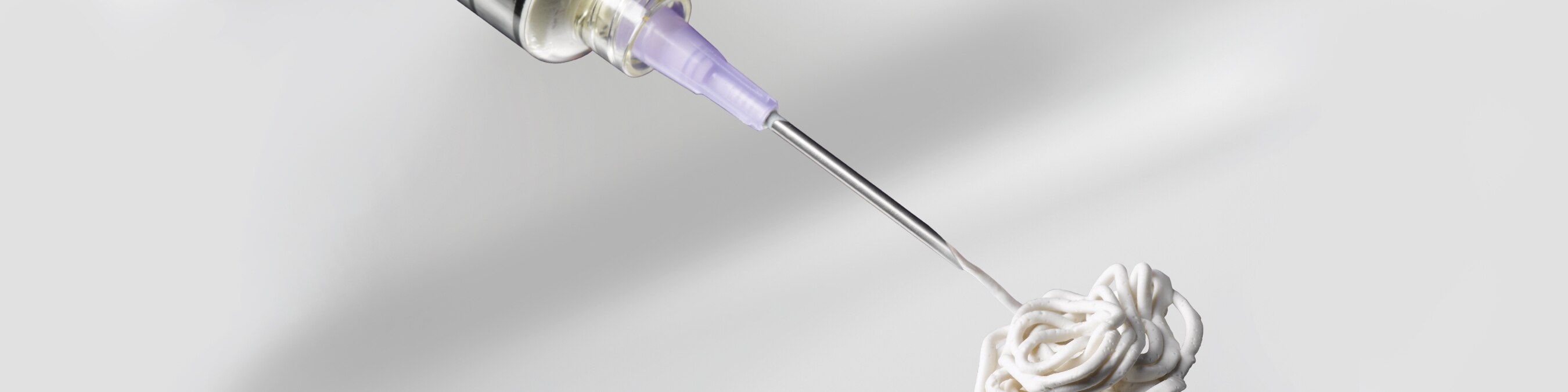
βBeta-bsm is injectable osteoconductive material for use in minimally invasive procedures. This synthetic, biocompatible bone graft substitute material sets hard and maintains shape at body temperature. The material is mixed in a closed syringe-to-syringe mixing system to minimize the potential for spills, and can be delivered through a needle as small as 20g.
- Provides a scaffold for new bone growth while undergoing cell-mediated remodeling as the bone heals1,2,3
- Nanocrystalline* calcium phosphate is comparable to the mineral composition of human bone1,4
- Injectable to allow hardening in a wet environment for minimally invasive procedures
- Provides 2 minutes of working time to allow for intraoperative flexibility
- Isothermally sets hard in 3-5 minutes to an average compressive strength of 30MPa5, three times strength of cancellous bone6
- Can be implanted in wet environment, irrigated while setting, and drilled after hardening7
- Available in single-patient, single-use kits in volumes of 2.5cc, 5cc