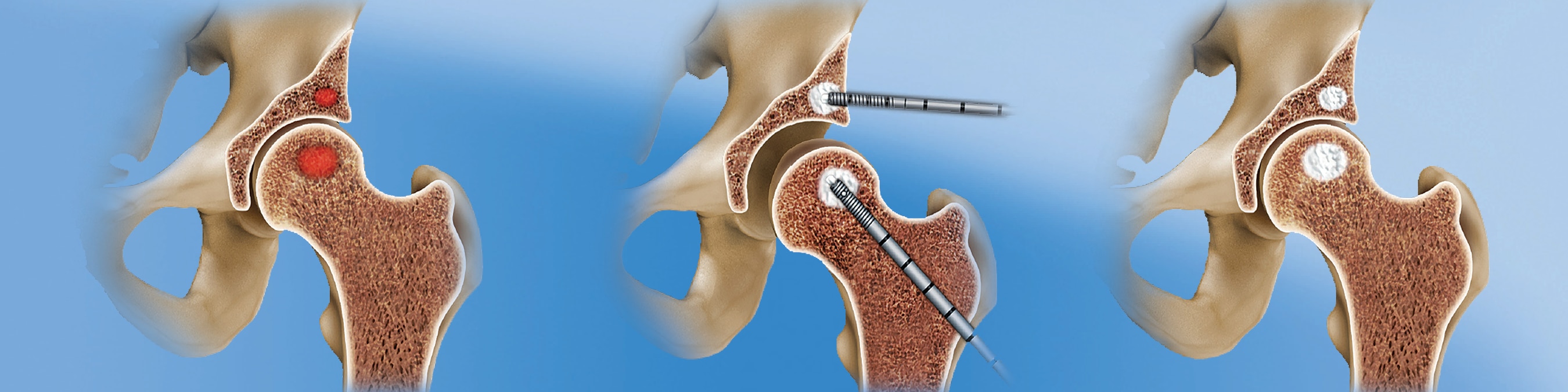
SCP for the hip is a minimally-invasive, fluoroscopically-assisted procedure that targets and fills subchondral bone defects in the acetabulum and femur through the delivery of AccuFill® Bone Substitute Material (BSM), a nanocrystalline, highly porous injectable calcium phosphate (CaP).
The SCP procedure in the hip is usually performed along with arthroscopy for visualization and treatment of findings inside the joint. In some cases, an open or mini-open procedure is necessary for access to the defect.