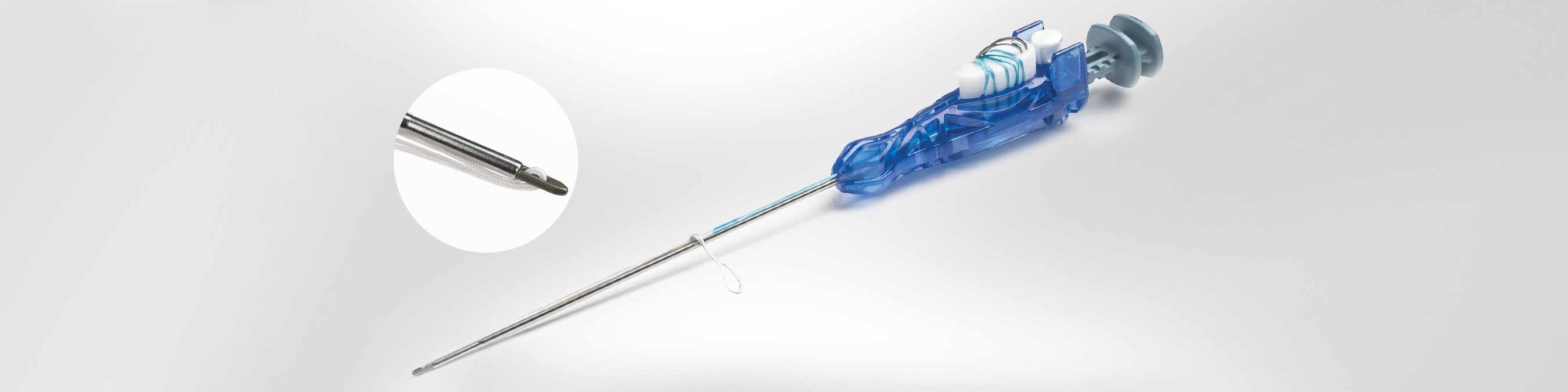
For those who desire a titanium button and adjustable loop fixation for a distal biceps repair, Zimmer Biomet has designed a NEW, low profile button on our ToggleLoc Fixation Device that fits through a 2.9 mm hole. This suspensory device incorporates button fixation and a knotless construct, which allows the tendon or graft to be easily zipped into the tunnel for final fixation.
A Simplified Technique
- The first button to be preloaded on an inserter handle for easy insertion
- Inserter handle incorporates implant, adjustable loop, and pusher/plunger function
Efficient
- Slotted reamer serves as the insertion guide, depending on technique preference
- Preloaded with needles for direct tendon attachment
Less Invasive
- Smallest drill hole on the market for suspensory fixation (button diameter is 2.9 mm x 10.3 mm)
- Interference
screw fixation is not necessary due to our ZipLoop Technology
- Interference screw can potentially damage the tendon upon insertion
- Allows for circumferential healing in the socket