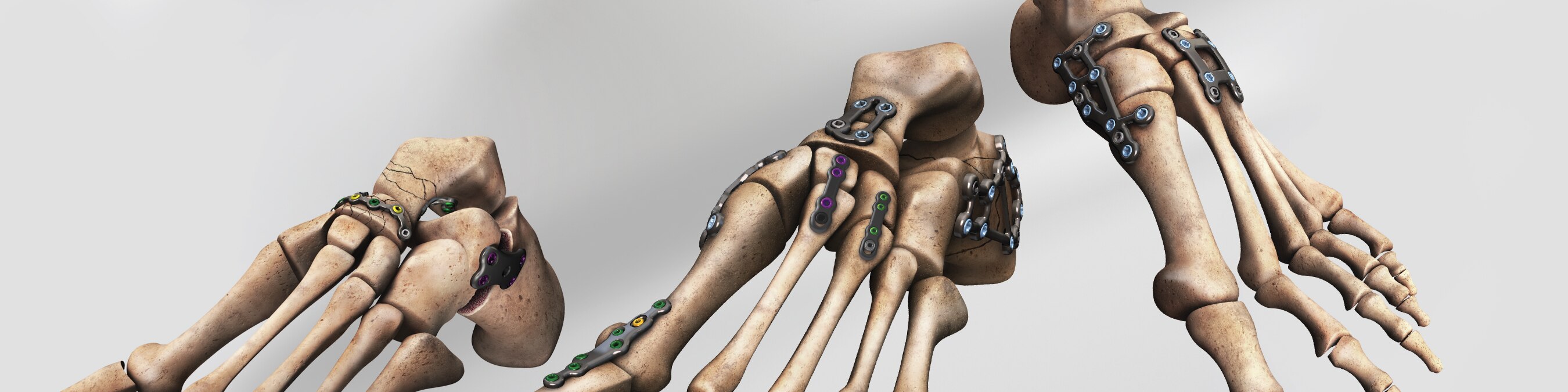
The A.L.P.S. Total Foot System offers a comprehensive, set of plating options that are anatomically contoured to address osteotomies, fusions, and fractures in the forefoot, midfoot, and hindfoot. Strategically designed regions of flexibility allow the surgeon to accommodate individual anatomic variations without compromising strength. The system also offers a wide array of locking and non-locking screw options that incorporate F.A.S.T. Guide® Technology. The broad, flexible system of implants and instruments provides practical, user-friendly solutions that help optimize O.R. efficiency.
- Intraoperative Flexibility for Customized Solutions
- F.A.S.T. Guide® Technology for O.R. Efficiency
- Comprehensive System of Plates, Screws, and Instruments,
including:
- Single Joint Fusion Plates
- Dorsal Midfoot Fusion Plates
- Lateral Column Lengthening Plates
- Lapidus Plate
- Medial Column Fusion Plate
- Locking Calcaneal Plate
- 1st Metatarsal Fusion Plates
- Talar Neck Fracture Plate
- Navicular Fracture Plate
- Web and Straight Plates
- Compression Wires
- Locking Multi-Directional Screws
- Low Profile Non-Locking Screws
- Low Profile Locking Screws